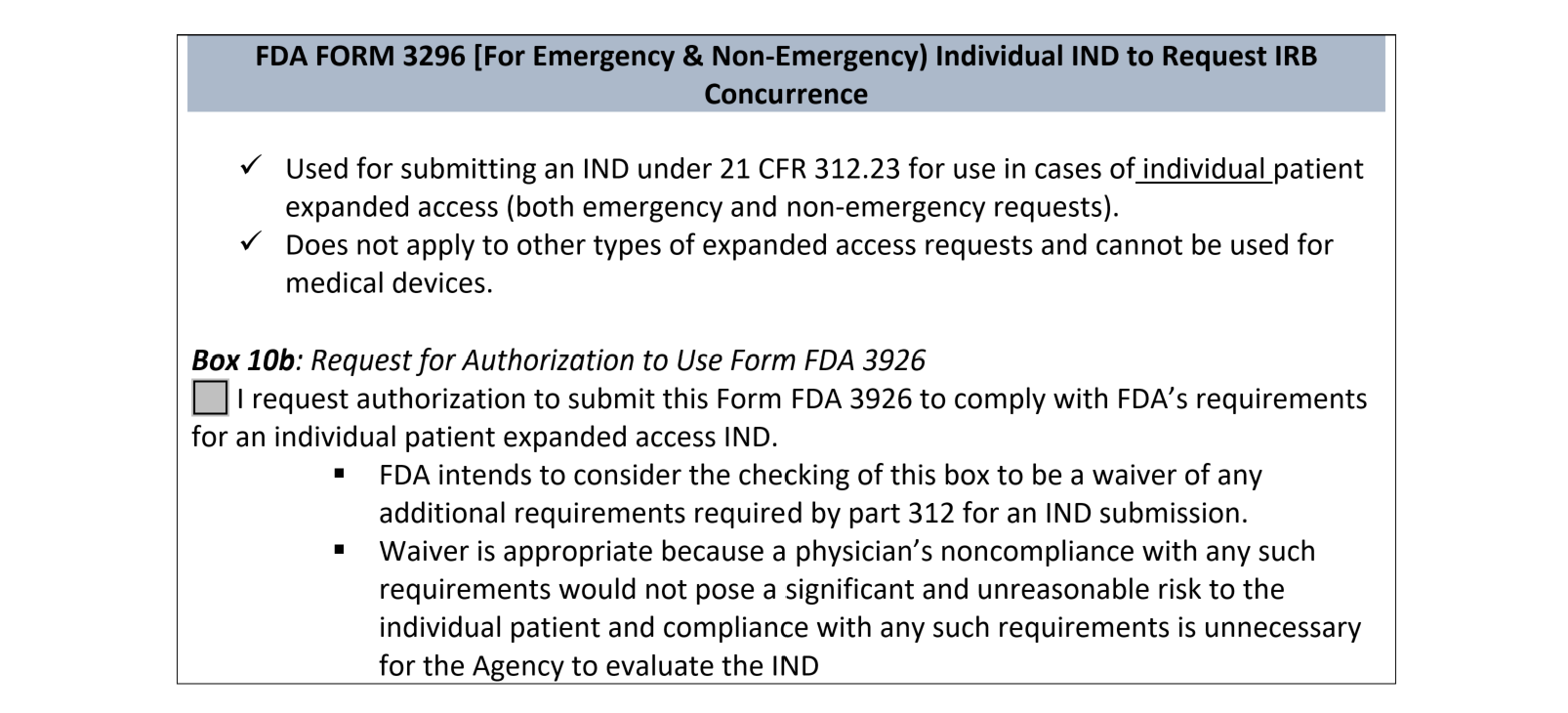
Single Use Expanded Access IND/IDE: FDA and IRB Requirements Before and After Use IRB Webinar October 9, ppt download

Once-daily, single-inhaler mometasone–indacaterol–glycopyrronium versus mometasone–indacaterol or twice-daily fluticasone–salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study - The ...
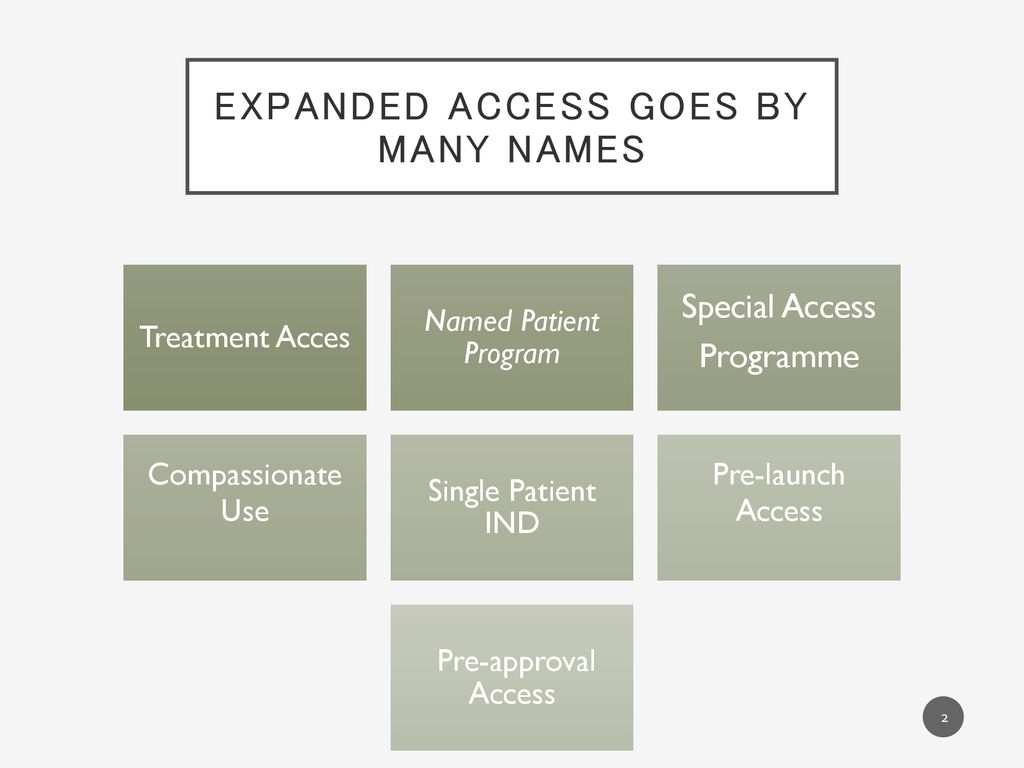
Intermediate-Size Patient Populations INDs: What Are They, When Should They Be Used, and Who May Apply for Them?” Richard Klein, Former Director, FDA. - ppt download
Access to Investigational Drugs: FDA Expanded Access Programs or â•œRightâ•'toâ•'Tryâ•š Legislation?

Individual Patient Expanded Access: Developing Principles For A Structural And Regulatory Framework | Health Affairs

Retrospective evaluation of single patient investigational new drug (IND) requests in pediatric oncology - Shulman - 2021 - Cancer Medicine - Wiley Online Library




![INVESTIGATIONAL NEW DRUG [IND] APPLICATION SUBMISSION (1).pdf INVESTIGATIONAL NEW DRUG [IND] APPLICATION SUBMISSION (1).pdf](https://image.slidesharecdn.com/investigationalnewdrugindapplicationsubmission1-221128141339-adad00bd/85/investigational-new-drug-ind-application-submission-1pdf-27-320.jpg?cb=1669645214)