Eversense sensor placement. (a) The positioning guide for the Eversense... | Download Scientific Diagram

Implantable glucose sensor featuring IDT sensing technology awarded CE Mark - Medical Design and Outsourcing

Senseonics keeps busy with Europe launch, Roche partnerships while FDA wait continues | MobiHealthNews

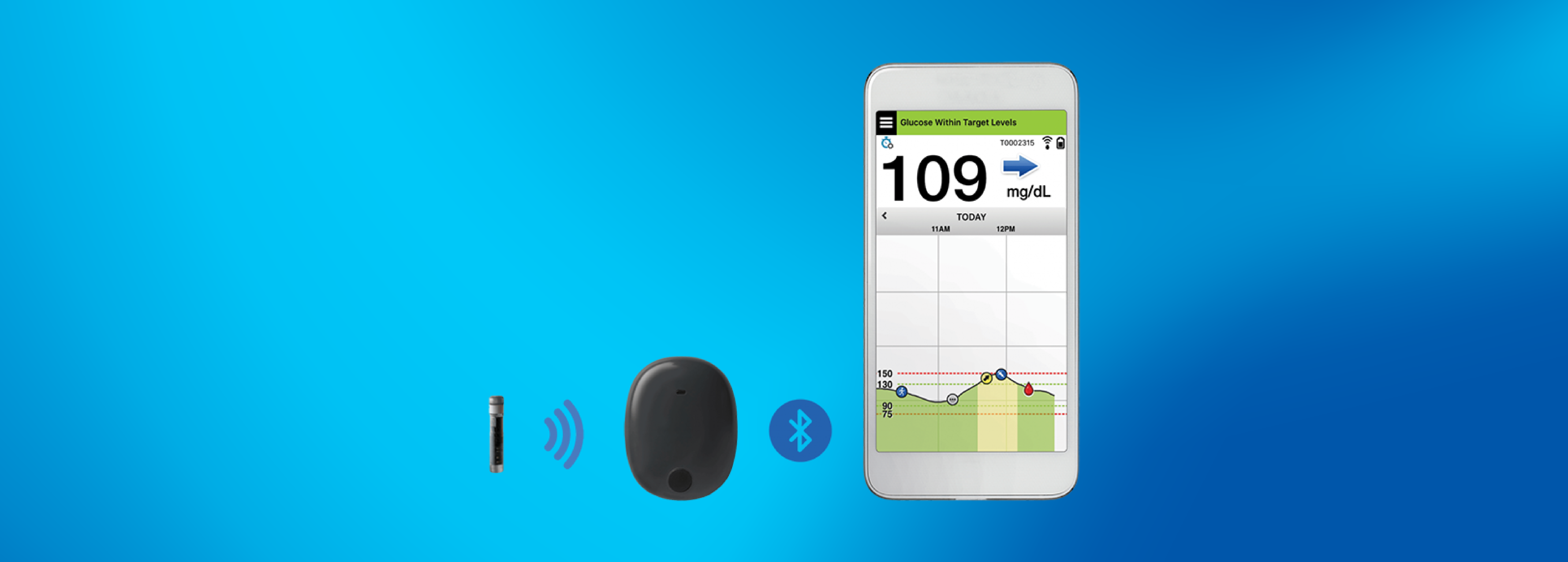
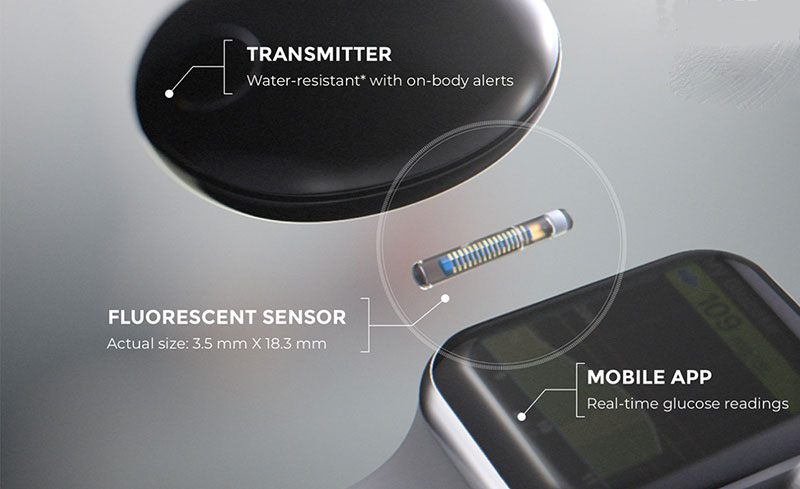
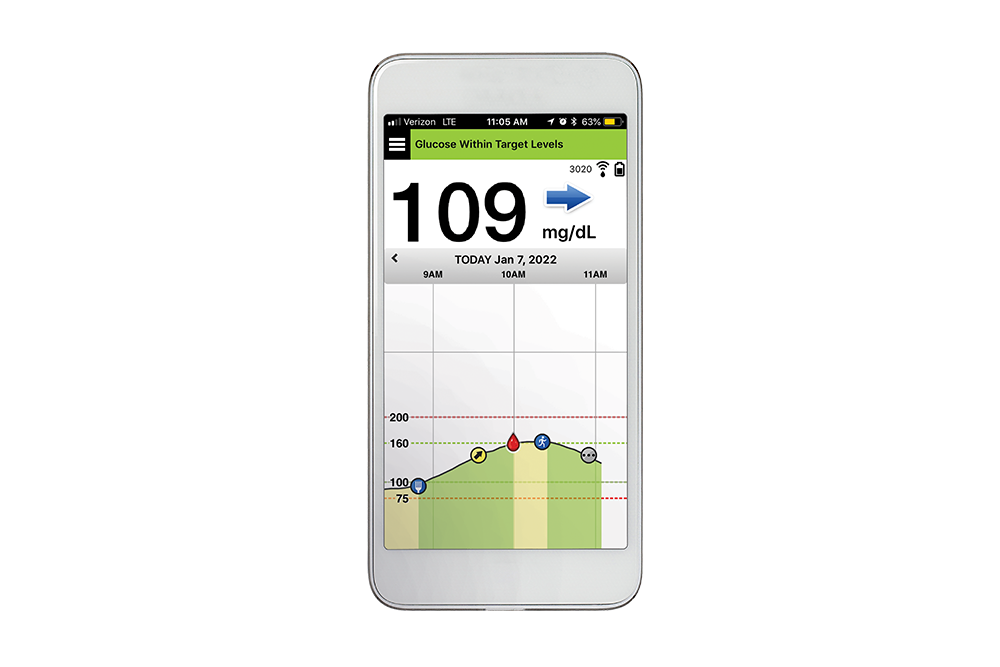
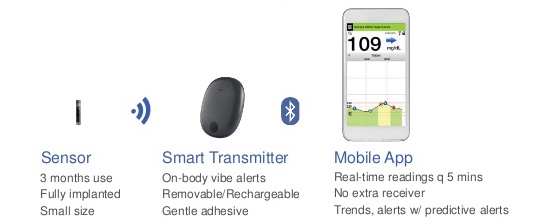
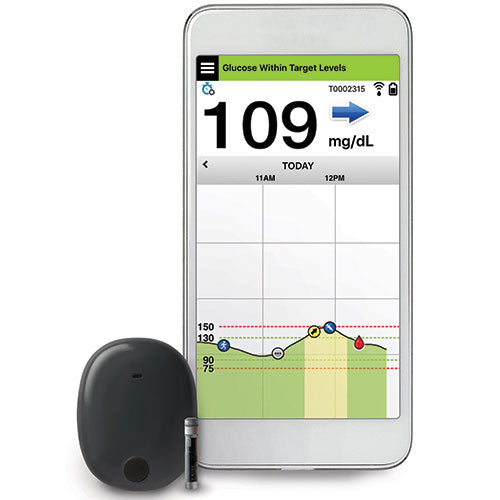
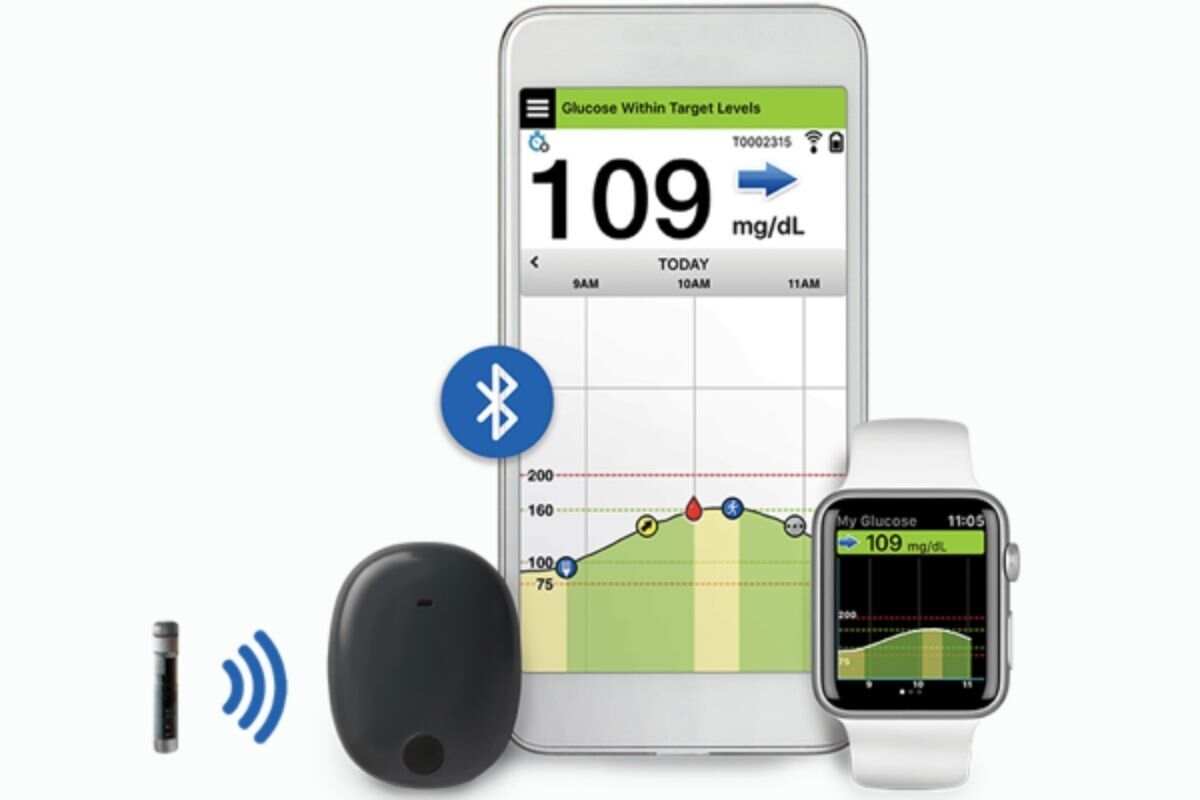
Beyond Type 1 - NOW APPROVED BY THE FDA - a new CGM is on the scene! The Eversense from Senseonics is the first implantable continuous glucose monitor. It lasts 90 days